What Are Hemiacetal
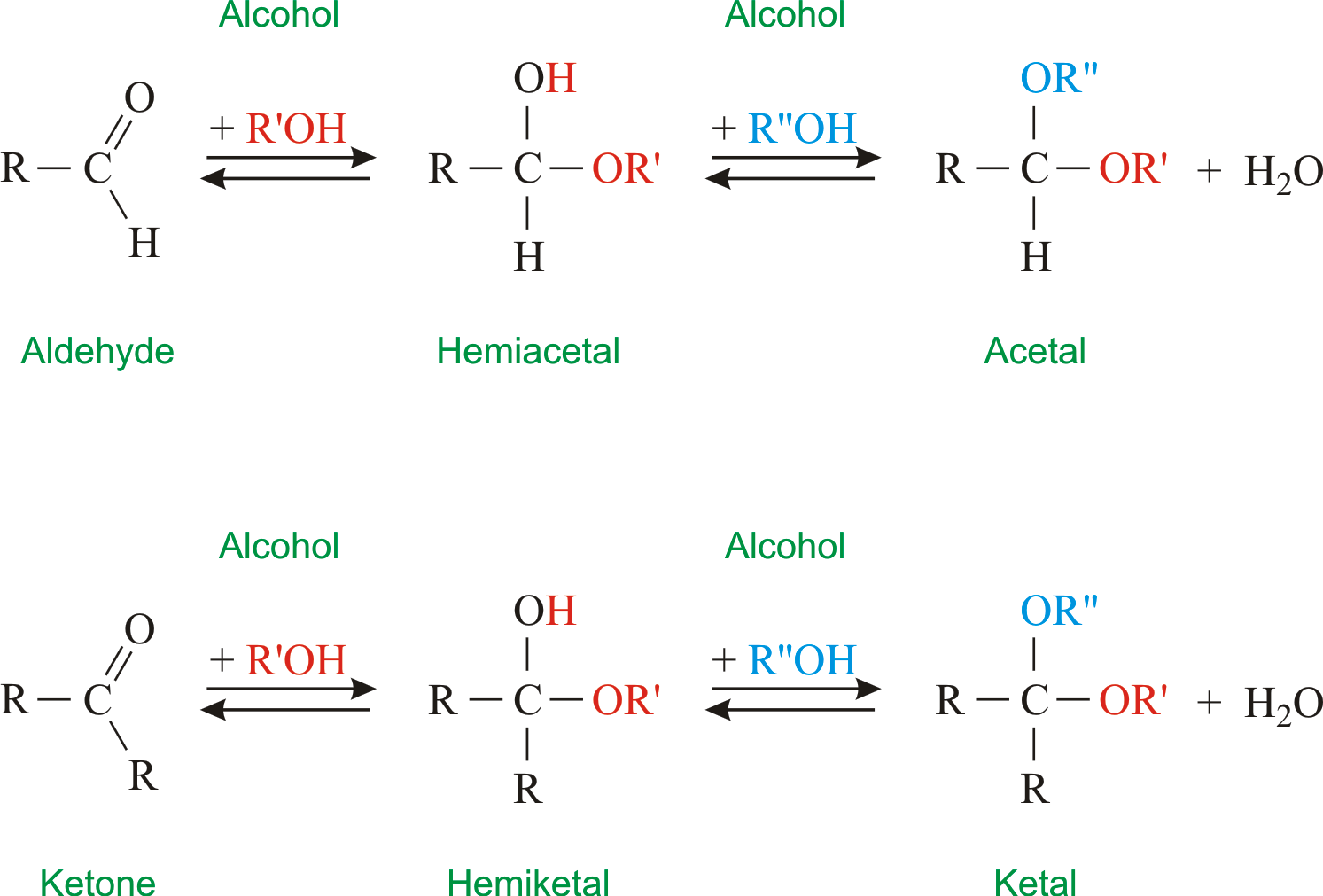
Hemiacetals and acetals are important functional groups because they appear in sugars. The prefix hemi half is used in each term because as we shall soon see addition of a second alcohol nucleophile can occur resulting in species called acetals and ketals.
 10 3 Hemiacetals Hemiketals And Hydrates Chemistry Libretexts
10 3 Hemiacetals Hemiketals And Hydrates Chemistry Libretexts
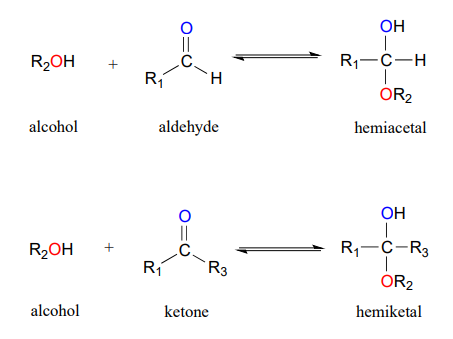
If one molecule of aldehyde RCHO reacts with one molecule of alcohol R1OH a hemiacetal is formed RCH OHOR1.
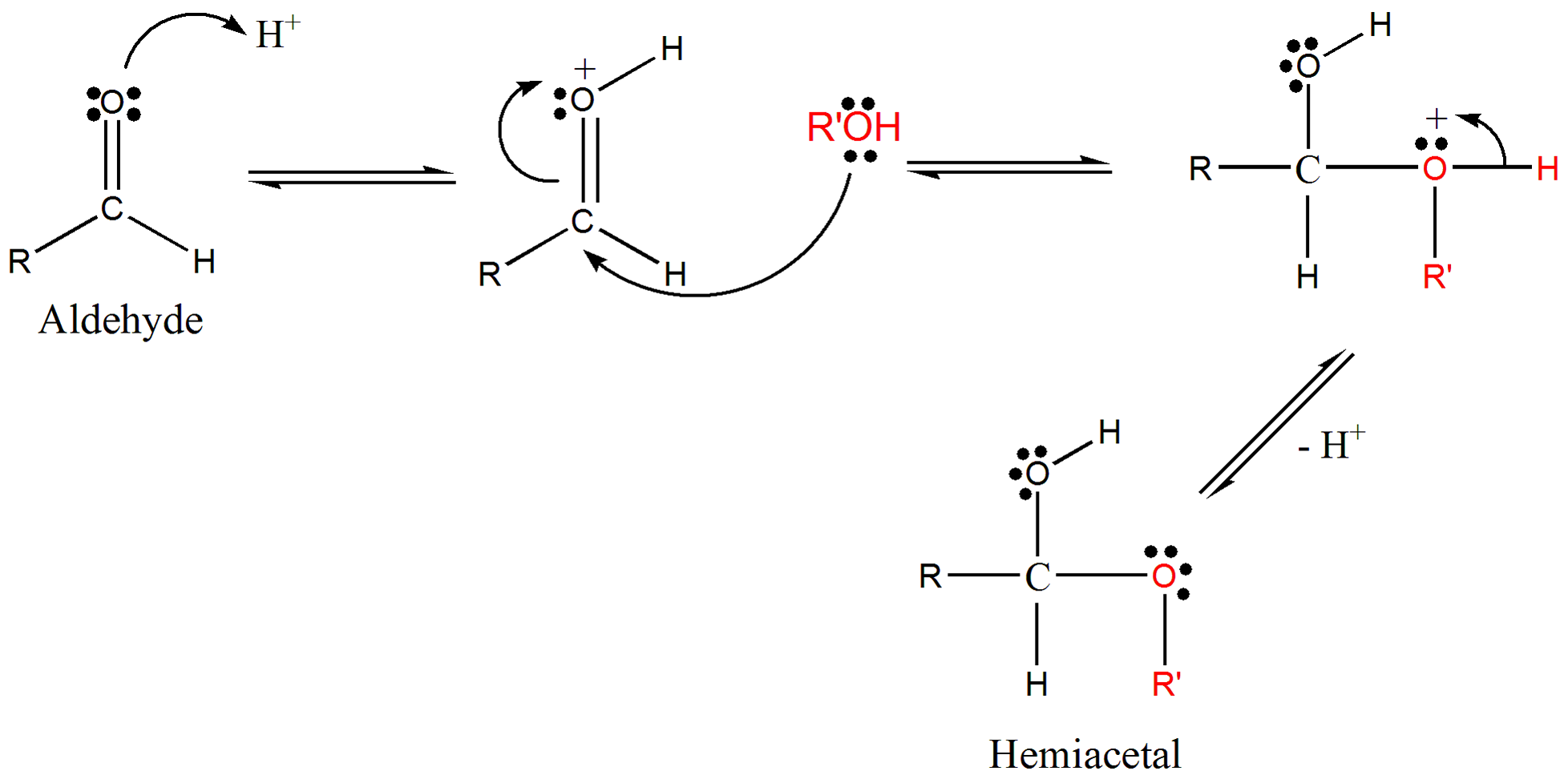
What are hemiacetal. When an alcohol adds to a ketone the resulting product is a hemiketal. Since alcohols are weak nucleophiles the attack on the carbonyl carbon is usually promoted by protonation of the carbonyl oxygen. Further reaction with a second alcohol molecule produces a full acetal RCH OR12.
A hemiacetal is derived from an aldehyde. A hemiacetal is a carbon connected to two oxygen atoms where one oxygen is an alcohol OH and the other is an ether OR. Hemiacetal is formed from aldehydes.
If it is formed from a ketone then it is called a hemiketal. Acetals hemiacetals ketals and hemiketals in drug metabolism The equilibrium between the carbonyl forms of aldehydes or ketones and their associated acetalhemiacetal or ketalhemiketal forms also plays a critical role during the bodys metabolism of xenobiotics drugs. A Hemiacetal is formed from an aldehyde.
Here the R groups can be organic fragments a carbon atom with arbitrary other atoms attached to that or hydrogen while the R groups must be organic fragments not hydrogen. An OR group -OH group -R group and a H group. The CO bond formed is a glycosidic bond and the OR from the alcohol is called an aglycone.
Created by JayWatch the next lesson. In contrast if this compound is formed from a ketone then it is called a Hemiketal. To achieve effective hemiacetal or acetal formation two additional features must be implemented.
When this reaction takes place with an aldehyde the product is called a hemiacetal. The two R groups can be equivalent to each other a symmetric acetal or not a mixed acetal. Any of a class of compounds characterized by the grouping C OH OR where R is an alkyl group and usually formed as intermediates in the preparation of acetals from aldehydes or ketones.
In detail the central carbon atom in both of these compounds is a sp 3 -C atom bonded to four bonds and out of these four bonds only one bonding type is different. However for formation of five or six numbered rings in an intra-molecular hemiacetal formation the equilibrium is actually to the right. Acetal compounds are formed by addition of alcohol molecules to aldehyde molecules.
Hemē-kētăl A product of the addition of an alcohol to a ketone. The hemiacetal and hemiketal forms of monosaccharides react with alcohols to form acetals and ketals called glycosides. The hemiketal forms of the sugars are involved in polysaccharide formation as glycosyls or glycosides.
Remember that R is short hand to denote any carbon chain. -OR group -OH group -R group and H group. And so this is a very important reaction.
Hemiacetal is an intermediate chemical compound formed during the chemical process of acetal formation. Hemiacetals are generated from an aldehyde or ketone and one molecule of an alcohol with the formation of one ether bond and an OH group to the same carbon atom from the carbonyl group. The fourth bonding position is occupied by a hydrogen.
A hemiacetal is an alcohol and ether ATTACHED TO THE SAME CARBON. Hemiacetal is a group of atoms composed of a central carbon atom bonded to four groups. Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon but have substantially.
In the ketose sugars the hemiketal formation is from an attack by an internal OH on the ketone carbonyl leading to intramolecular cyclization furanose or pyranose. Therefore these two groups have a slight difference in their chemical structure. A hemiacetal or a hemiketal is a compound that results from the addition of an alcohol to an aldehyde or a ketone respectivelyThe Greek word hèmi meaning half refers to the fact that a single alcohol has been added to the carbonyl group in contrast to acetals or ketals which are formed when a second alkoxy group has been added to the structure.
Hemiacetal is an organic compound in which a central carbon atom is bonded with four different groups. How aldehydes or ketones react with alcohols to form hemiacetals or hemiketals. Pyrolysis of Organic Molecules Second Edition 2019.
So the formation of hemiacetals usually the equilibrium is actually favors the formation of your aldehyde or ketones so its usually back here to the left. An acetal is a functional group with the connectivity R 2 C OR 2. And when this reaction takes place with a ketone the product is referred to as a hemiketal.
The general formula of a hemiacetal is given as RHC OHOR. When an alcohol adds to an aldehyde the result is called a hemiacetal. The chemical formula of Hemiacetal is RHC OHOR.
 Functional Groups Flashcards Quizlet
Functional Groups Flashcards Quizlet
 What Is A Hemiacetal Formation Definition Video Lesson Transcript Study Com
What Is A Hemiacetal Formation Definition Video Lesson Transcript Study Com
 Hemiketal Chemistry Dictionary Glossary
Hemiketal Chemistry Dictionary Glossary
 Hemiketal An Overview Sciencedirect Topics
Hemiketal An Overview Sciencedirect Topics
 21 3 Formation Of Hydrates Hemiacetals Acetals Organic Chemistry Ii
21 3 Formation Of Hydrates Hemiacetals Acetals Organic Chemistry Ii
 Hemiacetals Acetals Flashcards Quizlet
Hemiacetals Acetals Flashcards Quizlet
 Acetal Ketal Hemiacetal Hemiketal Reaction Overview And Shortcut Youtube
Acetal Ketal Hemiacetal Hemiketal Reaction Overview And Shortcut Youtube
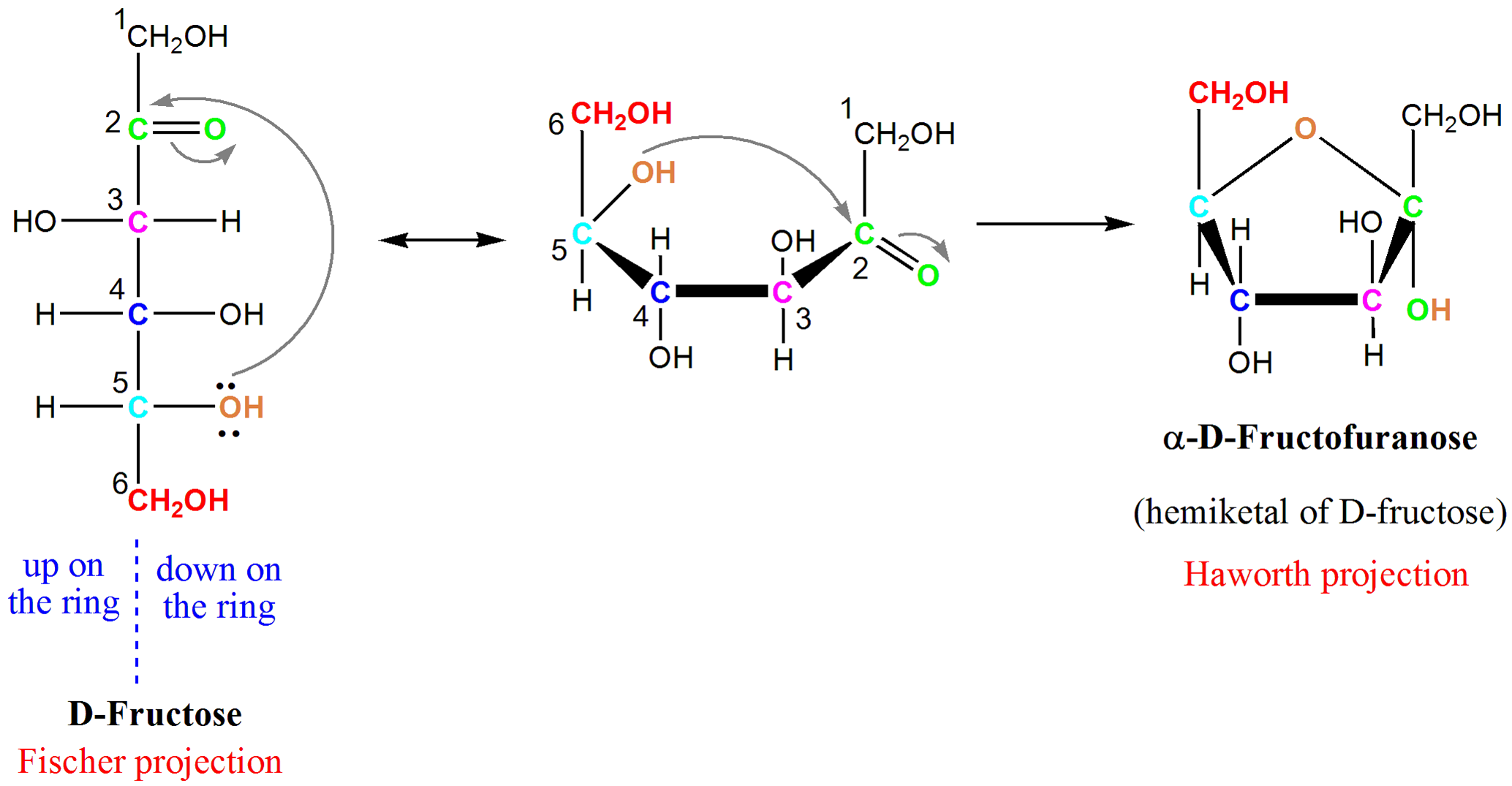 Cyclic Hemiacetals And Hemiketals Article Khan Academy
Cyclic Hemiacetals And Hemiketals Article Khan Academy
 What Is A Hemiacetal Formation Definition Video Lesson Transcript Study Com
What Is A Hemiacetal Formation Definition Video Lesson Transcript Study Com
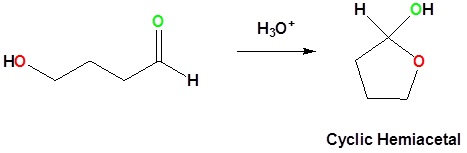 21 16 Cyclic Hemiacetals Chemistry Libretexts
21 16 Cyclic Hemiacetals Chemistry Libretexts
 The Key Difference Between Hemiacetal And Hemiketal Is That Hemiacetal Is Formed Via The Reaction Between An Alcohol And Chemistry Organic Chemistry Different
The Key Difference Between Hemiacetal And Hemiketal Is That Hemiacetal Is Formed Via The Reaction Between An Alcohol And Chemistry Organic Chemistry Different
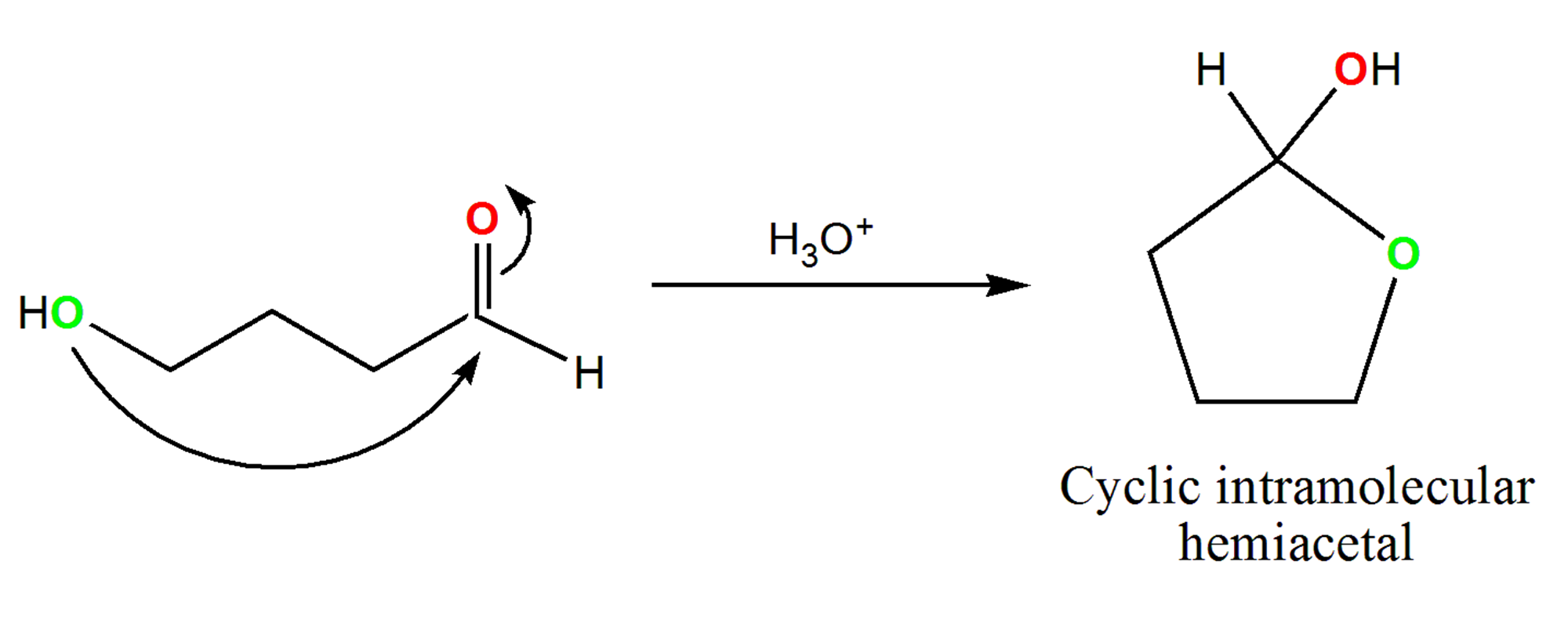 Cyclic Hemiacetals And Hemiketals Article Khan Academy
Cyclic Hemiacetals And Hemiketals Article Khan Academy
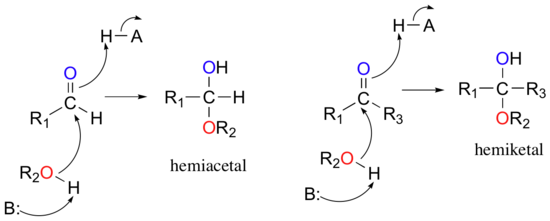 Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry
Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry
 Cyclic Hemiacetals And Hemiketals Article Khan Academy
Cyclic Hemiacetals And Hemiketals Article Khan Academy
 What Is A Hemiacetal Formation Definition Video Lesson Transcript Study Com
What Is A Hemiacetal Formation Definition Video Lesson Transcript Study Com
 Hemiacetal An Overview Sciencedirect Topics
Hemiacetal An Overview Sciencedirect Topics
